For adults with newly diagnosed Ph+ CML-CP
An innovative study of initial TKI treatment1
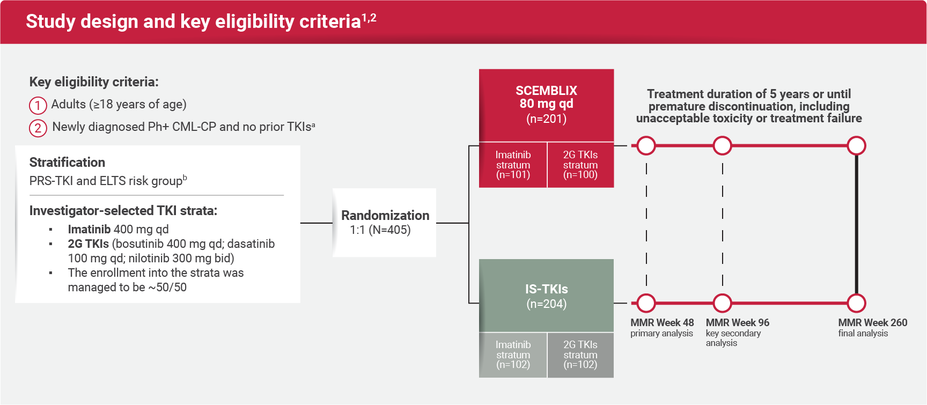
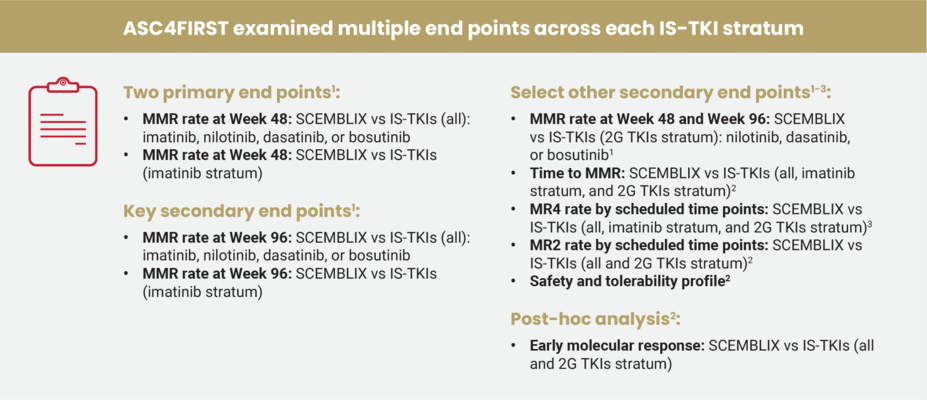
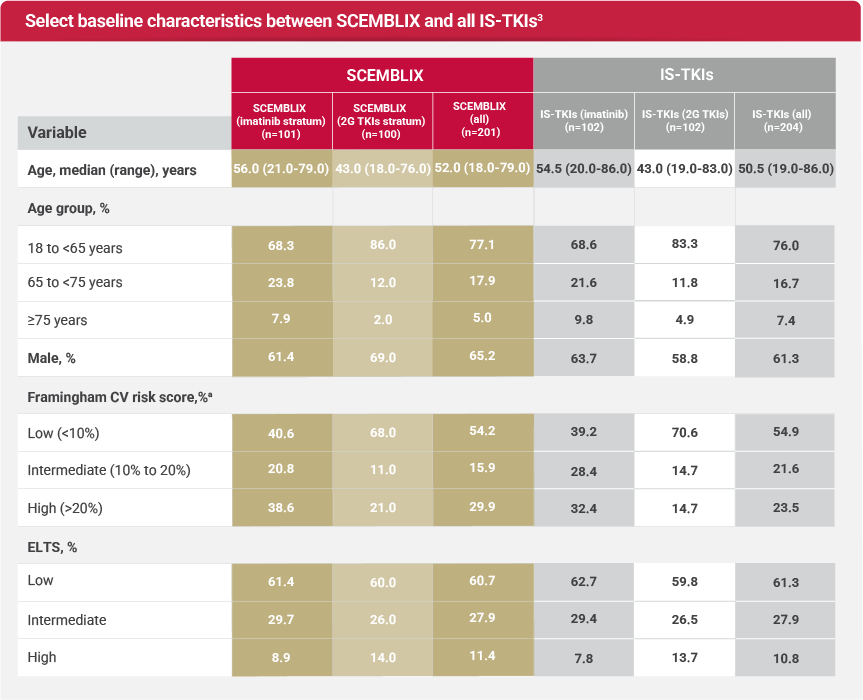
ASC4FIRST was a multicenter, randomized, active-controlled, open-label study where Investigators, in consultation with patients, preselected the appropriate TKI and evaluated patients' ELTS risk scores. Patients were then stratified by preselected TKI and ELTS score. Once stratified, they were randomized to receive either SCEMBLIX or an Investigator-selected TKI.1,2
aTreatment with any TKIs prior to randomization was not allowed, except for a period of ≤2 weeks of either imatinib, nilotinib, dasatinib, or bosutinib.2
bELTS groups included low, intermediate, and high.1