NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend asciminib (SCEMBLIX®) as a Category 1, Preferred first-line treatment option for newly diagnosed adult patients with Ph+ CML-CP across risk groups (low-, intermediate-, or high-risk score).†,‡
†NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
‡See the NCCN Guidelines® for detailed recommendations, including other preferred treatment options.
Imagine what is possible with SCEMBLIX
SCEMBLIX was studied vs all standard-of-care TKIs§ in patients with newly diagnosed Ph+ CML-CP in ASC4FIRST2,5,||
§Imatinib, nilotinib, dasatinib, and bosutinib.
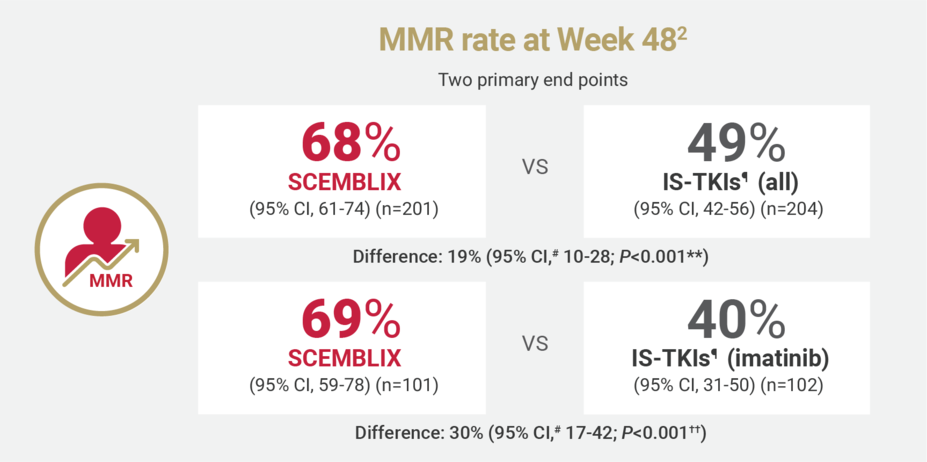
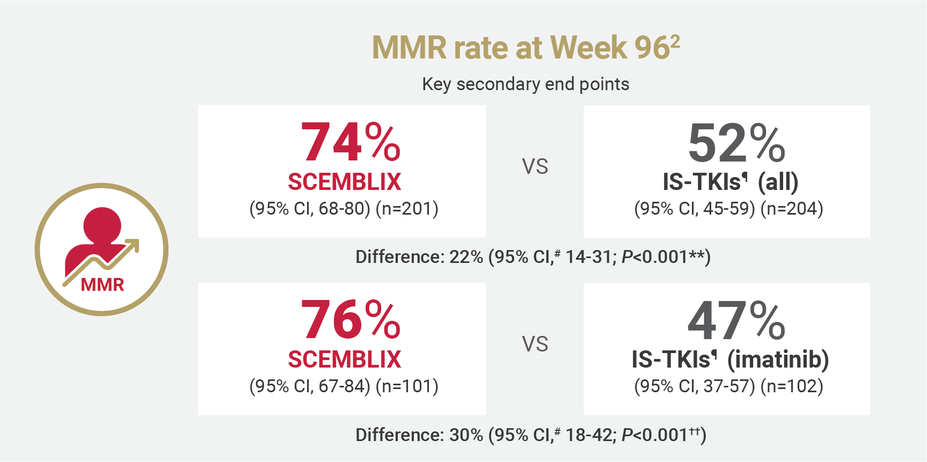
SCEMBLIX delivered superior response rates at Weeks 48 and 962
¶IS-TKIs included imatinib (400 mg once daily) and other TKIs of nilotinib (300 mg twice daily), dasatinib (100 mg once daily), or bosutinib (400 mg once daily).2
#Estimated using a common risk difference stratified by PRS-TKI and baseline ELTS risk groups.2
**Adjusted P-value using a Cochran-Mantel-Haenszel 1-sided test stratified by PRS-TKI and baseline ELTS risk groups.2
††Adjusted P-value using a Cochran-Mantel-Haenszel 1-sided test stratified by baseline ELTS risk groups.2
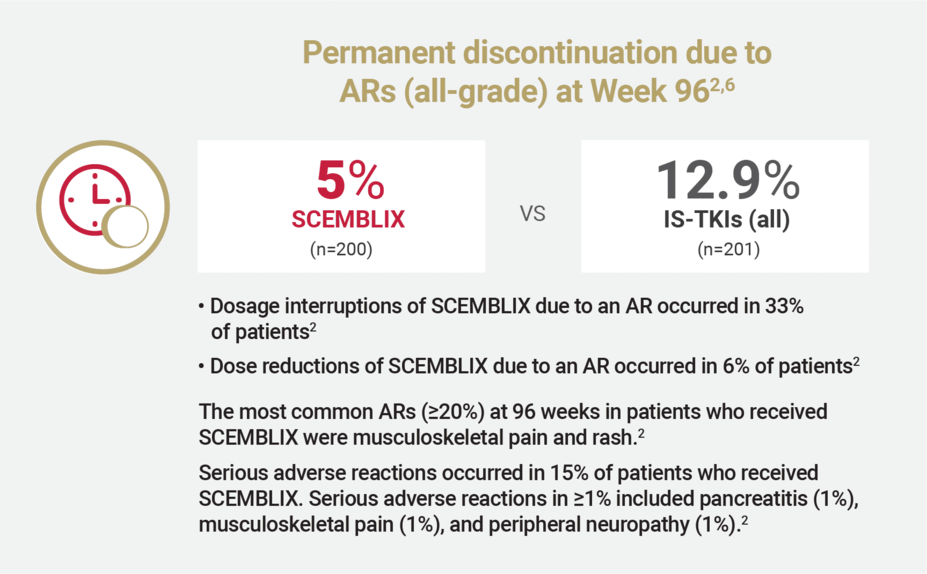
At Week 96, the discontinuation rate due to ARs was >2X lower with SCEMBLIX vs standard-of-care TKIs (all)2,6
AR, adverse reaction; CML, chronic myeloid leukemia; ELTS, EUTOS long-term survival; EUTOS, EUropean Treatment Outcome Study; IS-TKI, Investigator-selected tyrosine kinase inhibitor; MMR, major molecular response; NCCN, National Comprehensive Cancer Network; qd, once daily.
||ASC4FIRST is a multicenter, randomized, active-controlled, open-label study of 405 adults with newly diagnosed Ph+ CML-CP. Investigators, in consultation with patients, preselected the appropriate TKI and evaluated ELTS risk scores. Patients were then stratified by preselected TKI and ELTS score, then randomized (1:1) to receive either SCEMBLIX or an Investigator-selected TKI (imatinib, nilotinib, bosutinib, or dasatinib). 201 patients received SCEMBLIX at 80 mg qd, and 204 patients received IS-TKIs until unacceptable toxicity or treatment failure occurred.2,5